On a bookshelf in the office of Dr. Theodore Lawrence, head of radiation oncology at the University of Michigan Health System (UMHS), is a May 5, 1958, cover of LIFE magazine about new cancer treatment methods that shows a patient about to receive a beam of radiation. Draped in a white sheet and lying on a hospital treatment table, the patient is dwarfed by an x-ray machine the size of a medieval siege cannon.
University of Michigan Health System At a Glance
- An academic health system within a major public research university
- UMHS Hospitals and Health Centers
- 817 beds, 44,000 admissions
- 1.6 million out-patient visits, 100 clinics
- 18,000 employees
- UMHS Medical School
- 1,600 faculty physicians
- 1,000 resident physicians
- 700 medical students
Since then radiation technology has changed dramatically. Today’s machines, called linear accelerators, are much smaller and much more accurate. They deliver a very precise dosage to a small area of a patient’s body, killing cancer cells but harming fewer healthy cells. But the processes surrounding treatment have not kept pace. In fact, they often are overwhelmed by the increased complexities in technology and escalating demand for the latest treatment.
“Radiation therapy today is almost unrecognizable compared to 1958,” said Dr. Lawrence. “The ability to target and deliver radiation has increased amazingly. Processes, however, are almost the same.”
Most of the steps in radiation therapy revolve around three major processes. In order of occurrence they are: consultation with a radiation oncologist, simulation and planning of treatment, treatment. With the exception of a couple steps in planning, which now use computerized axial tomography (CAT) scans to create 3-D images of organs, the basic process today would be familiar to the Life patient. How long it took her to go from first step to the start of treatment is lost, but in mid-2005 as part of an effort to expand a lean transformation, a study by the UMHS radiation oncology department discovered that only 43% of new patients with bone or brain metastases were receiving consultation, simulation, and first treatment within a day. For most, first treatment required three visits to the hospital over five days and could take as many as 10 days. Typically, patients came in for a consultation, went home, came in for simulation, went home, and then came in for their first treatment.
For most, first treatment required three visits to the hospital over five days and could take as many as 10 days. Typically, patients came in for a consultation, went home, came in for simulation, went home, and then came in for their first treatment.
Beginning treatment as soon as possible is important for patients with brain or bone metastases, a condition in which cancer cells are spreading. Radiation therapy relieves pain and preserves bone and brain function. “This is a group of patients who really need help,” Dr. Lawrence explained. “They’re having changes in mental status from the brain metastasis, they’re having severe headaches, they’re having pain in the bone. Everybody in the department knows these are people who have to be treated as quickly as possible.”
The department already had found a way to begin treatment very quickly for another group of patients. People with spinal cord tumors began radiation therapy within hours after consultation.
“They need immediate treatment or they could suffer paralysis,” explained Dr. Lawrence. The accelerated procedure delivered treatment quickly, but it was inefficient because it relied on working around or expediting the normal steps in consultation through first treatment. He thought a fundamentally better process could be designed using lean principles. “We said, Let’s try to use lean thinking tools and philosophy to design a system for patients with brain and bone metastases where we don’t have workarounds or expediting.’ ”
Ultimately, a series of cross-functional lean improvement teams designed and implemented a much-improved process so that the percentage of patients receiving consultation, simulation, and first treatment on the same day rose from 43% to nearly 94%, a level the department has sustained. “We now do same-day treatment essentially 100% of the time for patients who want it. That is about 94% to 96% of our total patients,” said Kathy Lash, department director of operations.
Some of the biggest advancements came from relatively simple tools such as value-stream mapping and standardized work that improved communication between steps.
And the teams did it without hurling expensive new technology at problems. In fact, some of the biggest advancements came from relatively simple tools such as value-stream mapping and standardized work that improved communication between steps. These advancements helped the team achieve a second major goal set by department leadership: improve the morale and work life of department staff who were working longer hours due to an increasing patient workload and increasing complexity in treatment technology.
Background
The introduction of lean thinking at UMHS followed a path tread years earlier by many manufacturing companies: it began with a quality improvement effort.
“I’d say the quality improvement movement in healthcare began heating up in the 1980s,” said Dr. John Billi, associate dean for clinical affairs, University of Michigan Medical School, and associate vice president for medical affairs, University of Michigan. Government reports criticized healthcare for quality while industry complained about escalating costs. As hospital managements in the U.S. discovered the work of quality guru W. Edwards Deming, they began adopting total quality improvement efforts.
But the efforts came in “fits and starts,” said Dr. Billi. U.S. hospitals initiated scattered projects to improve an emergency department or operating room. After a few months of progress, systems often regressed to the old ways of working. Such efforts represented “improvement as a side activity not improvement as a basic business strategy,” said Dr. Billi. Furthermore, these early efforts often involved nurses and staff but not doctors, which hurt sustainability.
At UMHS, when a key leader of the quality effort left in the late 1990s, the effort lost steam. “We still continue to do quality improvement projects in every department but the concept of an overarching improvement program that was pervasive throughout the organization was lost,” said Dr. Billi.
Then in 2003 an executive from Virginia Mason Hospital and Medical Center in Seattle, WA, who was a UMHS alumnus, spoke at the school about Virginia Mason’s application of the Toyota Production System as an overarching business system to improve hospital processes. “I was personally very impressed, transformed you might say,” Billi recalled. That evening he went to a bookstore to buy Lean Thinking. For the next six months, he read about lean management and talked to “everyone in the country” applying lean principles to healthcare.
Dr. Billi’s research led him to a Michigan neighbor and a proposal. GM, burdened with burgeoning healthcare costs, offered to show UMHS managers and staff its lean efforts to help them learn the concepts. The car maker also loaned the hospital a group of experienced lean coaches to train UMHS staff and assist with six projects. Rather than create a separate kaizen promotion office, the hospital organized its lean effort under the existing quality improvement structure, called the Michigan Quality System, to integrate both activities to create an overarching continuous improvement philosophy.
The six lean projects gave UMHS a cadre of eight in-house coaches from a variety of backgrounds, including engineering, nursing, and quality. In addition to their regular duties, they helped hospital departments with lean projects. Besides valuable training, the early projects with GM also gave the hospital a desire to go solo. “The facilitation from GM gave us the advantage of using experienced facilitators,” said Dr. Billi. “But the disadvantage is that you can become dependent on the outside facilitators.”
Scoping a Pilot Project
Like Dr. Billi, Dr. Lawrence had been studying the application of lean principles to healthcare, particularly how it could help radiation oncology. “Radiation oncology really turns out to be a great lean test bed,” Dr. Lawrence said. We really have definable steps that are well laid out: we see the patient, plan the treatment, treat the patient.”
In July 2005, Dr. Lawrence and about 20 department managers and staff participated in a high-level kaizen workshop that targeted the entire department for improvement, which would have covered all types of patients and cancers. Afterwards, leadership decided to narrow the focus to a pilot project aimed at treating within 24 hours patients with brain or bone metastases, who represented 15% of the roughly 1,550 new patients treated annually by the radiation oncology department’s site at UMHS.
“We scaled down the initial project scope,” explained Lash. “We thought we would fix everything but realized the project was too broad. We decided to tackle some of the smaller pieces and learn more about lean tools as we went.” Tightening the focus of the project had several advantages:
Starting first treatment within 24 hours for this group of patients would relieve their pain, while preserving bone and organ function.
- A same-day start of treatment would decrease the number of visits patients had to make, an important factor for many patients who lived outside southeastern Michigan where UMHS is located.
- The medical information necessary to initiate same-day therapy would almost always be available because nearly 100% of patients with brain or bone metastases were referrals from UMHS physicians or clinics.
- Procedures for the planning and delivery of treatment to this group of patients were well-understood and accepted by department faculty and staff.
The pilot and subsequent work by lean teams also would help department leadership address the need to improve capacity and staff morale. Lash, who also is a registered radiation therapist (RTT), said today’s advanced radiation technologies have improved treatment but added time and complexity to the department’s workload that stressed capacity and, ultimately, staff.
Ten years ago, she noted, machines delivered radiation from one or two sides of a patient. The typical treatment session lasted about 15 minutes. Today’s radiation machines are controlled by multiple computers using imagery and lasers to deliver high energy x-rays from five or six angles synchronized, in some cases, to a patient’s breathing. The therapy is far more precise and spares more healthy tissue. But therapy sessions, which now last from 15 to 90 minutes, are far more complex and time consuming to setup.
By mid-2005, treatment often went beyond the time allotted for a patient, which meant that a radiation machine and its usual complement of two therapists were delayed in treating the next patient, an effect that snowballed through the daily schedule. This led to long delays for patients and long days for staff members, who often worked past the normal 6 p.m. quitting time until 9 or 10 p.m. to treat everyone scheduled. It also meant that physicists, who make sure the department’s five linear accelerators are available and capable of delivering a beam of radiation to the right tissue at the right dosage, had to wait until late at night or early in the morning to perform regular maintenance. The long and unpredictable hours caused low morale and turnover, especially among radiation therapists.
Radiation therapist Patrick Clark noted that many staff members had an hour drive home after leaving the hospital late at night, compared to working at medical clinics where work usually finished at 3:30 p.m. “After a couple of years that looks a lot more attractive,” he said.
Thus, the pilot project would be much more than an effort to improve efficiency or capacity. It was, as Lash noted, an attempt to learn “how do we change the culture to introduce standard work so our professional processes are stable.” In addition, based on what they had learned about lean management, leadership believed that easing the overburden on staff and equipment was essential to maintaining high-quality and safe care.
Understanding the Current State: Diagnosing the 7 Wastes
Here are examples of Toyota’s classic seven wastes from radiation oncology:
1. Correction
- Fixing an incomplete medical chart;
- Setting up a treatment room for a patient who is late or has canceled;
3. Motion
- Searching for charts;
- Walking to retrieve equipment from a different treatment room;
4. Material Movement
- Getting equipment from a different treatment room;
5. Waiting
- Patients waiting for a delayed treatment;
- Waiting for a doctor, radiation machine, or information;
- Unevenness in the arrival of patients and treatment plans;
6. Processing
- Making unnecessary movements in the treatment room;
- Redundant or unnecessary mental or physical work;
- Therapists scheduling patients for future appointments;
7. Inventory
- Obsolete forms;
- Information or material waiting in queue.
The department established a 16-member lean team representing all front-line service providers, including clerical staff, attending and resident physicians, nurses, radiation therapists, simulation therapists, administrators, support staff, and physicists.
Guided by lean management’s emphasis on understanding the current situation based on facts, rather than opinions or assumptions, the team developed over a 90-day period: a current-state value stream map of the existing process for treating patients with bone and brain metastases; a leaner, much-improved treatment process represented by a future-state map; and a detailed work plan that assigned members specific tasks with timelines for making the proposed new process illustrated by the value-stream mapping a reality.
The team calculated key metrics for the current-state map:
- Process time: the actual time it takes to complete an
- activity;
- Total lead time: the total elapsed time to complete an
- activity;
- First time quality: the probability that a patient will
- go through all individual process steps without
- encountering a quality-related problem;
- Process cycle efficiency: process time divided by
- total lead time to measure what percentage of time is
- spent in value-added and nonvalue-added activities.
- Nonvalue added activity, or waste, is any activity that
- consumes resources but creates no value from the
- perspective of the customer.
The resulting current-state map revealed lots of waste in a therapy process requiring 27 individual steps for consultation, simulation, and first treatment. Process time (or value-added time) for the whole process averaged little more than half a work day (290 minutes or 4.8 hrs.), based on a 10-hour day. However, the total lead time for patients to complete the entire process was often seven days in real time (10,000 minutes), resulting in a value-added proportion (process cycle efficiency) as low as 3% (290 divided by 10,000).
The team calculated that the entire process contained 7,825 minutes of waiting time between steps and that only 0.2% of the bone or brain metastases cases went through the entire process without the need for some sort of rework. Only 43% of the patients in the six months before the pilot project completed the entire series of steps in a day.
As it drew a leaner future state based on data and direct observations, the team and leadership zeroed in on problems to attack in order to create a process that would begin treating patients on the same day. Since the current-state map showed that radiation therapy had less than five hours of actual process time “we thought we should be able to deliver treatment in a day,” said Dr. Lawrence. “Certainly if we saw a patient before noon, we could deliver treatment by 6 p.m. if we got rid of the waste and focused on the process time.”
The team developed a leaner future-state map based on these key observations: A lack of standardized work meant information flow into and within the department was chaotic, leading to poor communication, low first-time quality, and delays triggered by searching for paperwork, equipment or the need to fix missing or inaccurate information, such as unsigned forms or wrong details in charts. This condition necessitated the practice, which is common to healthcare in general, of inspecting work as it was passed from one step to the next.
The leaner future state process performed consultation, simulation, and treatment within a day by making all needed information available at the start of the process, implementing standardizing work, and applying clear process guidelines. These actions cut overall process steps to 16, reduced process time to 225 minutes, shrank total lead time to a day, and created the potential to bring first-time quality up to 100%.
The leaner future state process performed consultation, simulation, and treatment within a day by making all needed information available at the start of the process, implementing standardizing work, and applying clear process guidelines. These actions cut overall process steps to 16, reduced process time to 225 minutes, shrank total lead time to a day, and created the potential to bring first-time quality up to 100%.
The team decided to begin implementation by developing standardized work for consultation, the first step. Subsequent lean teams developed standardized work for the two other major steps — simulation and first treatment — and attacked other problems surfaced by the value-stream analysis.
First Step: Consultation
The therapy process begins when the radiation oncology department receives requests for treatment, usually from other UMHS departments. During consultation, patients are examined by a radiation oncologist, who often is assisted by a resident doctor and a nurse, to confirm the diagnosis and discuss a treatment plan with the patient.
The initial lean team, along with the affected clerical and professional staff, developed a standard form, called a consultation scheduling form, to be used by referring hospital departments and radiation oncology doctors who received referral calls from other doctors. The form, which could be emailed or faxed to doctors outside UMHS, was explained to clerical staff members. It required such key information as:
- Patient name, address, social security number, gender, phone number, insurance coverage, and primary care physician’s name;
- Referring physician name, unique identity number, clinic name, office address, phone number, and contact name;
- Diagnosis (determines whom the patient will see in the radiation oncology department) ;
- Medical information, including:
- Office notes
- Pathology reports
- Radiology reports
- Surgery reports
- Laboratory results
- Treatment summary, if treated before
Besides using the new consultation form, the department implemented new standardized work that required radiation oncology schedulers to quickly check an online calendar to identify what doctors were available to see bone and brain metastases cases within 24 hours. Schedulers called patients to tell them what times were available.
When a consultation time was set for that day, the new standardized work directed schedulers to notify the nursing desk assistant, who notified the chief radiation therapist and billing department that a bone or brain metastases case had been added to the day’s schedule. If the referral came from a UMHS department, the nursing assistant also called radiology for the patient’s images and retrieved medical documents from the in-house database for the medical chart being prepared for consultation. The chart was prepared in a standardized format based on input from doctors.
During the course of establishing standardized work, the team realized that clerks needed training in medical terminology so they could recognize when referring doctors were describing brain or bone metastases cases that had to be scheduled immediately.
During the course of establishing standardized work, the team realized that clerks needed training in medical terminology so they could recognize when referring doctors were describing brain or bone metastases cases that had to be scheduled immediately. “We had to really educate them in medical terminology,” said Lash. “We had not allowed them to be the thinkers they really were. We had put them into robot mode.”
Simulation After consultation, a patient has his or her treatment planned and simulated, a step that needed overtime virtually every day to complete its schedule. So, after the first lean team completed its work on consultation, a second team tackled developing standardized work for this critical step. The main issues for this phase of the project were:
- Reduce the long waits patients often had in the waiting room before treatment because previous appointments ran late.
- Keep treatments to the allotted times so delays don’t mount, extending operating hours.
- Improve employee satisfaction by working normal operating hours.
The team received valuable help in observing actual work and gathering additional data from several University of Michigan engineering students familiar with lean principles. Team members, including the engineering students, observed treatments at each of the five radiation machines, focusing primarily on the duties radiation therapists performed. They also observed front desk clerks scheduling consultations and staff and doctors working on the simulation step. This familiarized everyone with the overall process of delivering therapy so they could break it into logical tasks, which were recorded on the current-state map along with important data, such as cycle times and first-time quality.
The next step took nearly three weeks. Beginning in early October 2006, team members observed 20 patients during their first three treatment sessions. The team selected these first sessions because they set the course of subsequent therapy sessions, which could continue for several weeks. With clipboards and observation sheets in hand, team members followed patients from the time they arrived, noting when each task began, when it ended, and if any rework, equipment problems, or unusual incidents occurred. They observed staff performing such tasks as: educating patients about the treatment procedure, helping patients onto treatment tables, checking medical charts, activating the radiation beam, recording dosages given, helping patients out of the treatment room, and scheduling the next appointments.
The team also distributed surveys to radiation therapists, dosimetrists (who calculate the radiation dosage prior to treatment), clerks, and physicists to discover what employees thought caused delays. After analyzing the surveys, team members conducted follow-up interviews with a sample group of employees to clarify the top causes.
During simulation, a patient lies very still on an examining table while a radiation therapist uses a special x-ray machine to define the exact place on the body where the beam will be aimed. Simulation may use CAT scans or other imaging studies to help the oncologist and therapist plan how to direct the radiation. The simulation may result in some changes to the treatment plan to spare the greatest possible amount of healthy tissue from receiving radiation.
The body area receiving radiation is marked with a “tattoo,” tiny dots made by a temporary or permanent marker showing where to aim the beam. Depending on the type of treatment, the radiation therapist may make body molds or other devices that keep the patient from moving during treatment. These are made from foam, plastic mesh, or plaster. In some cases, the therapist will make shields that cannot be penetrated by radiation to protect organs and tissues near the treatment field.
Information from simulation, along with the radiation oncologist’s guidelines about radiation dosage, is downloaded to computers in a planning room, located near the treatment rooms, where dosimetrists use formulas to calculate radiation dosages.
The initial study had noted that a lot of information needed by simulation was communicated verbally by phone or in notes resulting in miscommunication, rework, lots of checks to catch the miscommunication, and when missing information caused delays “people pointing their fingers at each other,” said Lash.
Typical delays included: patients arriving at simulation with unsigned consent forms, patients arriving late because they were delayed by a prior chemotherapy treatment, and therapists calling doctors to check information. Radiation therapists had a basic idea of how doctors wanted patients setup for treatment based on the type of cancer. They’d page doctors for verbal instructions to find out if there were exceptions, for example, if a patient couldn’t lie down. If the doctor was with another patient, simulation could be delayed.
The team first devised a few easy-to-use standardized forms to obtain the information that simulation needed and that subsequent steps needed from simulation. The documents, essentially checklists, were designed to make sure necessary information was collected and conveyed simply and clearly from step to step — from referral call to consultation, from consultation to simulation, from simulation to first treatment, and from first treatment to scheduling subsequent treatments. It’s an approach Dr. Lawrence called “focusing on the white space.
“A lot of people want to burrow into how they do their step,” he explained. “We do some of that, but most of our gains have not been inside the steps, they’ve been in getting rid of the white space, the handoffs. It’s how do you get from step A to step B, not so much what happens inside the space, although we have had some nice gains there too.”
What follows are descriptions and then samples of the main forms designed and refined over many months by lean teams working on consultation, simulation, and first treatment:
- Patient Activity Documents collect key information during consultation that is needed by subsequent steps. Doctors and patients simply check boxes corresponding to standard procedures and write in any exceptions. The front of the form is for doctors and therapists to enter information about treatment (see page 11). Patients complete a basic medical history on the back (page 12).
- Physician Simulation Orders (page 13), also are filled out by doctors during consultation. They give therapists at the next step — simulation — essential information, such as whether or not the treatment area has to be immobilized. If a doctor wants the standard setup, he or she simply checks a box on the form. If there is an exception to the standard setup, for example, if the patient can’t lie down, the doctor writes in the new instructions. “It goes through a logical order of what we want to do with the patient, we sign the form, and it’s done. Today’s work is done today,” said Dr. Lawrence. He added that the simulation orders also are good teaching tools for doctors to use with assisting residents. After starting by creating Simulation Orders for brain and bone metastases cases, the department developed forms covering treatment for 90+ cancer types, such as breasts and esophagus. For example, the Simulation Orders and Planning Directive below are for breast treatments.
- Treatment Planning Directives (page 14) completed by doctors after simulation, this form goes to the planning room where dosimetrists use it with formulas to calculate dosages.
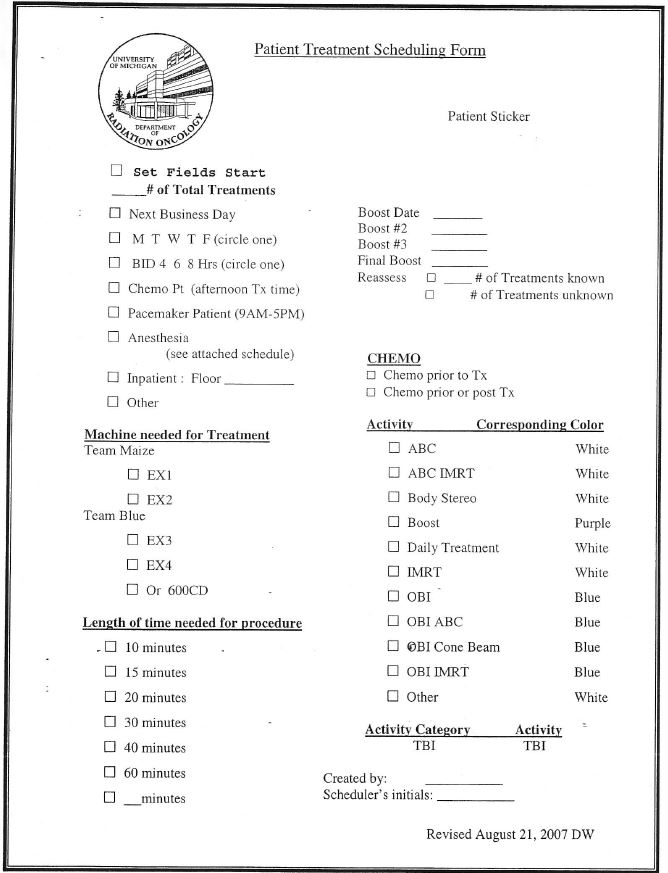
- The Patient Treatment Scheduling form (page 15) is filled out by radiation therapists and goes with the patient after first treatment to a centralized scheduling office where a scheduler establishes the rest of the treatment schedule.
Spreading the Transformation Under the umbrella of the “Michigan Quality System,” the University of Michigan Health System uses lean thinking concepts to create a consistent approach to quality and process improvement. Besides the application of lean principles in radiation oncology, other lean projects include:
- The Emergency Department used value-stream mapping to cut 10 minutes off the patient discharge process, which allowed two extra patients to be admitted daily. And ED second and third year residents now must do problem-solving projects using the 5Ss the 5 whys.
- Managers and senior managers are using A3 reports to foster dialogue and build consensus for solving problems, making proposals, and reporting status in budgeting, strategic planning, IT planning, operating room management, ambulatory care improvement, inpatient discharge, and other areas.
- In the Catheterization Lab a lean team standardized the activation process to reduce the “door to balloon” time from when a patient arrives to when a balloon or stent is inserted to clear a blocked artery. The lab averaged 72 minutes in 2007, compared to the American Heart Association’s national goal of 90 minutes.
- The Pathology Lab, using work flow analysis and material handlers (“water spiders”) following set routes at regular periods to collect blood samples, reduced the time to deliver samples from 32 minutes to nine. Other improvements cut analyses turnaround times by 38%, travel distances by 33%, and waiting times by 78%.
- At the new Cardiovascular Center one of 17 lean projects underway standardized surgical preparation processes in the Electrophysiology Lab so surgical patients are properly prepared for the procedure 100% of the time.
“You want to make the forms very simple and look for the exceptions,” explained Lash, who said the forms enhance safety. “I tell people all the time when they say, ‘Cathy why do we have to use the standardized forms or do any more lean projects?’ The bottom line is for safety.”
First Treatment
The last form — Patient Treatment Scheduling — represented a breakthrough in how radiation therapists worked. The major tasks for therapists, who usually worked in teams of two but sometimes three, were basically to help a patient onto the treatment table and into any immobilization devises. Then, from a control console outside the treatment room’s heavy steel doors, they used computers and imagery to align the radiation beam with the patient’s tattooed treatment area, and activated the radiation beam. After treatment, a therapist helped the patient off the table, then the other therapist would schedule the patient’s next appointments. Therapists also were responsible for taking calls from patients who wanted to reschedule appointments. In the earlier survey, therapists had identified scheduling as their leading cause of delays, which was supported by observations.
Scott Hadley, a clinical physicist and member of the team, said, “As we collected data and watched what went on, everyone noticed that after first treatment a therapist sat at a computer with the patient spending 15 or 20 minutes trying to schedule the next several weeks of treatment, working around vacations, work, or chemotherapy appointments while the other therapist needed help in the treatment room getting the next patient started.”
“We decided to standardize scheduling and take it out of the control room, which keeps the therapist focused on treating patients and puts the scheduling in the hands of people who are really good at scheduling,” said Lash, who developed guidelines for a central scheduling clerk to follow. The new Patient Treatment Scheduling form (above) gave the scheduler key information for developing a realistic schedule, including the type of machine needed for treatment, time needed for treatment, and whether or not the scheduler must coordinate radiation therapy with the chemotherapy department.
“Taking scheduling out of the control room solved so many problems. We stopped the chaos that it caused and patients liked it much better. It’s a little more comforting for them to come into the scheduling room away from the treatment area.”
“Taking scheduling out of the control room solved so many problems,” said Lash. “We stopped the chaos that it caused and patients liked it much better. It’s a little more comforting for them to come into the scheduling room away from the treatment area,” she said.
Maize and Blue
The initial lean team also had called attention to data it collected during work observations showing that the times for therapists to perform the same tasks ranged from 1 to 7 minutes. The discrepancies, due to inconsistencies in procedures, made it difficult to accurately predict how much time was needed for treatments.
“We saw that there was no standard work for who was doing what,” Lash said. Department leadership, along with therapists, developed two sets of standardized work with primary and secondary tasks. One set is for therapists working in a treatment room helping patients onto the treatment table and into an immobilization device. The other is for a therapist assigned to the control console preparing the program, aligning the machine, calling up patient records, and reviewing patient charts for additional instructions. (Standardized work also was developed for when there are three therapists assigned to a machine. See sample blank forms below.)
Each day, therapists are assigned to a specific team and wear a colored shirt (maize or blue for University of Michigan colors) to identify whether they are at the console or treatment room.
Having standardized work ensured that each treatment followed the same procedure and that a specific person was accountable for completing each step accurately. The standardized work advanced continuous improvement. By having therapists administer treatment identically, areas of waste became easier to identify, countermeasures became easier to develop, and the effectiveness of the improvements became easier to track.
Billing Guidelines
By January 2007, oncology department leadership was extending the lean transformation beyond the main treatment processes to supporting tasks, such as video teleconferencing, purchasing, and billing, where mistakes in coding bills were causing a lot of rework.
When staff members delivered any procedures to patients, such as x-rays, they entered billing codes on patients’ medical charts. Codes for complicated procedures such as simulations and actual radiation treatments varied with levels of complexity and radiation dosages.
Chief radiation therapists and supervisors audited every patient’s medical chart at the end of treatment to ensure all charges were captured and that billing codes were correct. On virtually every chart, they found errors such as missing codes or wrong codes that needed correction. Lash said few inaccurate charts ever went to insurance companies for payment, but the mistakes, auditing, rework, and correcting the wrong bills that did slip out, all resulted in a major time sink for her and her supervisors, who reviewed at least 1,600 charts annually for an average of 20 minutes per chart.
After training in lean concepts and studying how the billing process worked, a lean team identified root causes for the problems preventing 100% first time quality, including:
- Lack of training for new employees.
- Lack of training on the computer system used to enter codes.
- Interruptions due to heavy patient volume.
- Information about coding mistakes was not being fed back to staff. “They didn’t know they were doing anything wrong because we never told them. We just fixed the charts,” said Lash.
Over the course of nine months, the team developed billing guidelines then ran training sessions for everyone entering codes. The training is given to new employees and repeated annually for everyone entering codes. The billing software was changed to make it more user-friendly and to catch potential errors by prompting anyone entering a charge not usually associated with a step. Finally, supervisors fed information about errors that they caught back to staff members. With supervisors now spending just four minutes auditing charts for correct codes, the department is much closer to its goal of eliminating auditing, Lash said.
A Different Kind of Kaizen
The billing lean team, like the earlier teams in radiation oncology, continues to meet to improve the process they “own” and check that past improvements are sustained. Billing is one of six cross-functional lean teams currently operating in the department. While their approaches to training, data collection, mapping, and use of lean tools would be recognized by Lean Thinkers in any service or industrial company, teams have had to modify their kaizen workshop design to accommodate the realities of a hospital environment. Unable to shut down the department or even an area for several days to implement improvements, as is the norm during most kaizen workshops, radiation oncology teams meet every other week for two to two-and-a-half hours to further the kaizen process.
“In between meetings we have homework to do, such as data collection, that we report on at the next meeting,” explained Lash, who serves on each team and often gets involved in data collection and implementation in order to tie the entire departmental effort together. Intervals between meetings are used to implement improvements approved at team meetings.
Reactions to Change
“I was resistant to some of the methods because I didn’t understand how they would help until we picked a process, worked on it, and I got to see it all the way through. Then I recognized that this would be really good for all of us.”
– Melanie Hamilton,
Radiation Therapist
As improvements took root and processes improved, initial skepticism about lean thinking withered.
“In the beginning I wasn’t always the easiest one to work with because I really didn’t understand the reasons,” said radiation therapist Melanie Hamilton, who admits she wasn’t fond of collecting data. “In fact I was resistant to some of the methods because I didn’t understand how they would help until we picked a process, worked on it, and I got to see it all the way through. Then I recognized that this would be really good for all of us.”
And staff also quickly recognized the benefit of lean process improvements for patients. “If you can take somebody who is in pain and instead of five days to get started we can treat them on the first day, that’s wonderful,” said radiation therapist Phil Zegarowski.
As continuous improvement becomes the normal operating procedure, Lash is beginning to see her role shift from traditional management based on firefighting to lean management based on problem solving and mentoring. “I love problem solving,” she said, “and I’ve always loved it, but I used to problem solve by putting out fires, but they would pop up somewhere else. Now when someone comes to me with a problem, they know they should have already asked the five whys. I help guide and mentor people so I’ve become more of a coach rather than a firefighter.”
In the early days of the department’s lean effort she saw her workload increase as she was transitioning between firefighting and coaching. “Now I’m starting to feel the relief,” she said.
Getting Docs on Board
Dr. Lawrence said the lean transformation also is changing his approach to managing. “I used to think that a chairman’s job was to give answers. It isn’t. These people know their work better than I do. What I’ve learned is that I’m good at asking questions but not good at giving answers.”
He also learned that big projects aren’t always needed for big gains. “We made some initial errors in trying to scope projects that were too large. So now what we do is develop value-stream maps, identify small problems, and pick them off. Find a bottleneck, fix it — okay what’s the next bottleneck? Form little teams, do little things, but remember that those little things incrementally pile up. As you pile up a bunch of successes, people get the spirit.” Including doctors.
Doctors initially resisted the lean effort, arguing that “we’re a great department why do we have to change?” or “the current process works for me,” Dr. Lawrence recalled.
“I kept telling them, ‘I’m not telling you how to practice. We’re just standardizing how you’re going to communicate.’ “But in an academic research organization, standard work is a complex undertaking. We hire the best researchers and now we’re telling them to be standardized. You have to show them that if they do this work in a standard way, it will free up time for research.” He also signaled to staff in leadership positions to serve on lean teams. “It’s now part of being a leader in this department.”
A big breakthrough in attitudes as well as process improvement came with the successes of the brain and bone metastases pilot and subsequent improvements in the treatment planning process. Doctors and staff realized the lean effort wasn’t an attempt to change care giving but the processes supporting it. Now the department is poised for another breakthrough, according to Dr. Lawrence.
As a result of studying the work in the simulation step from a lean thinking perspective, improvement team members realized that patients requiring very sophisticated planning and patients requiring simple planning could be separated. New simulation software packages and some additional training will allow radiation therapists to plan the simple cases, giving highly trained dosimetrists more time to concentrate on the complex cases and saving time for patients with simple planning needs.
“This is a real job challenge for the therapists,” said Dr. Lawrence, “but they are excited about it because it will help them grow professionally. And it will off-load a task from the dosimetrists.”
When the lean transformation began two-and-a-half years ago, radiation oncology was treating about 120 patients daily until 9 p.m. or 10 p.m. Now it treats the same or more by 6 p.m. Besides the resulting benefits of greater capacity, speedier treatment for patients, and less stressful days for staff — all of which were expected — Dr. Lawrence was surprised by an unexpected benefit.
“What I didn’t anticipate were the benefits of a calmer atmosphere and how that would improve staff morale.”
“What I didn’t anticipate were the benefits of a calmer atmosphere and how that would improve staff morale,” he said. “Chaos just drains your energy. When you feel like you have to redo things and develop workarounds, it takes the peaks out of our day. It drains your energy when you feel like you are fighting the system. Being able to treat this number of patients and not have to redo work — you can just feel the morale improve. Things are calm.”
And a relapse to the old way of working doesn’t appear likely. “I see a lean process in everything now,” said Dr. Lawrence. “We’ll do this forever.”
More Information:
- University of Michigan Health System — UMHS includes three hospitals, approximately 40 health centers, 120 outpatient clinics, the U-M Medical School and its Faculty Group Practice, the U-M School of Nursing and the Michigan Health Corp. UMHS was honored by the American Hospital Association for highest quality care and patient safety. UMHS was the lone finalist for the AHA-McKesson Quest for Quality Prize. U-M Hospitals and Health Centers was named one of “America’s Best Hospitals” for the 13th year in a row and received recognition for excellence in 15 areas of specialized care in the 2008 U.S. News and World Report.
- The Michigan Quality Health System — MQS is part of the continuing effort to improve quality, safety, efficiency, and appropriateness across the U-M Health System’s three missions of patient care, education, and research.
- See also: Presentation by James Womack, founder and chairman, Lean Enterprise Institute: “Lean Thinking: From Factory to Health Care & Tools to Management”
Intro to Lean Thinking & Practice
An introduction to the essential concepts of lean thinking and practice.